<Back to Index>
- Chemist Robert Wilhelm Eberhard Bunsen, 1811
- Geologist and Mineralogist Alexandre - Emile Béguyer de Chancourtois, 1820
PAGE SPONSOR


Robert Wilhelm Eberhard Bunsen (30 March 1811 – 16 August 1899) was a German chemist. He investigated emission spectra of heated elements, and discovered caesium (in 1860) and rubidium (in 1861) with Gustav Kirchhoff. Bunsen developed several gas - analytical methods, was a pioneer in photochemistry, and did early work in the field of organoarsenic chemistry. With his laboratory assistant, Peter Desaga, he developed the Bunsen burner, an improvement on the laboratory burners then in use. The Bunsen – Kirchhoff Award for spectroscopy is named after Bunsen and Kirchhoff.
Robert Bunsen was born at Göttingen in 1811 in what is now the state of Lower Saxony in Germany, but was then the short lived Kingdom of Westphalia; upon the defeat of Napoleon three years later it became the Kingdom of Hanover. Robert was the youngest of four sons of the University of Göttingen's chief librarian and professor of modern philology, Christian Bunsen (1770 – 1837). Sources disagree on Robert Bunsen's exact birth date. His parish register, as well as two curricula vitae, handwritten by Bunsen himself, support the claim that 30 March 1811 is Bunsen's true birth date; however, many later sources cite 31 March as the date. According to his biographer Georg Lockemann, Bunsen himself celebrated his birthday on the 31st in his later years. Lockemann nevertheless regarded the 30th as the correct date.
After attending school in Holzminden, Bunsen matriculated at Göttingen in 1828 and studied chemistry with Friedrich Stromeyer as well as mineralogy with Johann Friedrich Ludwig Hausmann and mathematics with Carl Friedrich Gauss. After obtaining a Ph.D. in 1831, Bunsen spent 1832 and 1833 traveling in Germany, France, and Austria; Friedlieb Runge (who discovered aniline and in 1819 isolated caffeine), Justus von Liebig in Gießen, and Eilhard Mitscherlich in Bonn were among the many scientists he met on his journeys.
In 1833 Bunsen became a lecturer at Göttingen and began experimental studies of the (in)solubility of metal salts of arsenous acid. His discovery of the use of iron oxide hydrate as a precipitating agent is still today the most effective antidote against arsenic poisoning. In 1836, Bunsen succeeded Friedrich Wöhler at the Polytechnic School of Kassel. Bunsen taught there for three years, and then accepted an associate professorship at the University of Marburg, where he continued his studies on cacodyl derivatives. He was promoted to full professorship in 1841.
Bunsen's work brought him quick and wide acclaim, partly because
cacodyl, which is extremely toxic and undergoes spontaneous combustion
in dry air, is so difficult to work with. Bunsen almost died from arsenic poisoning, and an explosion with cacodyl cost him sight in his right eye. In 1841, Bunsen created the Bunsen cell battery, using a carbon electrode instead of the expensive platinum electrode used in William Robert Grove's electrochemical cell. Early in 1851 he accepted a professorship at the University of Breslau, where he taught for three semesters.
In late 1852 Bunsen became the successor of Leopold Gmelin at the University of Heidelberg. There he used electrolysis to produce pure metals, such as chromium, magnesium, aluminium, manganese, sodium, barium, calcium and lithium. A long collaboration with Henry Enfield Roscoe began in 1852, in which they studied the photochemical formation of hydrogen chloride from hydrogen and chlorine. He discontinued his work with Roscoe in 1859 and joined Gustav Kirchhoff to study emission spectra of heated elements, a research area called spectrum analysis. For this work, Bunsen and his laboratory assistant, Peter Desaga, had perfected a special gas burner by 1855, influenced by earlier models. The newer design of Bunsen and Desaga, which provided a very hot and clean flame, is now called simply the "Bunsen burner".
There had been earlier studies of the characteristic colors of heated elements, but nothing systematic. In the summer of 1859, Kirchhoff suggested to Bunsen that he try to form prismatic spectra of these colors. By October of that year the two scientists had invented an appropriate instrument, a prototype spectroscope. Using it, they were able to identify the characteristic spectra of sodium, lithium, and potassium. After numerous laborious purifications, Bunsen proved that highly pure samples gave unique spectra. In the course of this work, Bunsen detected previously unknown new blue spectral emission lines in samples of mineral water from Dürkheim. He guessed that these lines indicated the existence of an undiscovered chemical element. After careful distillation of forty tons of this water, in the spring of 1860 he was able to isolate 17 grams of a new element. He named the element "caesium", after the Latin word for deep blue. The following year he discovered rubidium, by a similar process.
In 1860, Bunsen was elected a foreign member of the Royal Swedish Academy of Sciences.
Bunsen was one of the most universally admired scientists of his generation. He was a master teacher, devoted to his students, and they were equally devoted to him. At a time of vigorous and often caustic scientific debates, Bunsen always conducted himself as a perfect gentleman, maintaining his distance from theoretical disputes. He much preferred to work quietly in his laboratory, continuing to enrich his science with useful discoveries. As a matter of principle he never took out a patent. He never married.
When Bunsen retired at the age of 78, he shifted his work solely to geology and mineralogy, an interest which he had pursued throughout his career. He died in Heidelberg, aged 88.

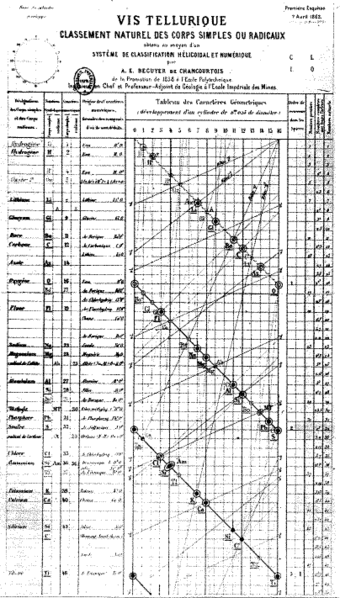
Alexandre - Emile Béguyer de Chancourtois (20 January 1820 – 14 November 1886) was a French geologist and mineralogist who was the first to arrange the chemical elements in order of atomic weights, doing so in 1862. De Chancourtois only published his paper, but did not publish his actual graph with the proposed arrangement. Although his publication was significant, it was ignored by chemists as it was written in terms of geology. It was Dmitri Mendeleyev's table published in 1869 that became most recognized. De Chancourtois was also a professor of mine surveying, and later geology at the École Nationale Supérieure des Mines de Paris. He also was the Inspector of Mines in Paris, and was widely responsible for implementing many mine safety regulations and laws during the time.
De Chancourtois was born in 1820 in Paris. At age eighteen, he entered the renowned École Polytechnique, one of the best known French grandes écoles of engineering and management. While he was there, de Chancourtois was a pupil of three famous French scientists, Jean - Baptiste Élie de Beaumont, Pierre Guillaume Frédéric le Play, and Ours - Pierre - Armand Petit - Dufrénoy. After completing his studies at École Polytechnique, de Chancourtois went on a geological expedition into Hungary, Armenia and Turkey. In 1848, de Chancourtois went back to Paris and joined the teaching faculty as professor of mine surveying at the École Nationale Supérieure des Mines de Paris. He worked with le Play to organize a collection of minerals for the French government. In 1852, De Chancourtois was named the professor of geology at École Nationale Supérieure des Mines de Paris. In 1867, de Chancourtois was awarded the Légion d'honneur by Napoleon III of France.
De Chancourtois led several overseas expeditions during the course of
his life and served as the Inspector of Mines in Paris from 1875 until
his death. As a mine inspector, he introduced safety laws to prevent
methane gas explosions, which were frequent occurrences at the time. He died in 1886 in Paris.
In 1862, a year before John Alexander Reina Newlands published his classification of the elements, de Chancourtois created a fully functioning and unique system of organizing the chemical elements. His proposed classification of elements was based on the newest values of atomic weights obtained by Stanislao Cannizzaro in 1858. De Chancourtois devised a spiral graph that was arranged on a cylinder which he called vis tellurique, or telluric helix because tellurium was the element in the middle of the graph. De Chancourtois ordered the elements by increasing atomic weight and with similar elements lined up vertically.
A.E.B. de Chancourtois plotted the atomic weights on the surface of a cylinder with a circumference of 16 units, the approximate atomic weight of oxygen. The resulting helical curve, which de Chancourtois called a square cirlcl triangle, brought similar elements onto corresponding points above or below one another on the cylinder. Thus, he suggested that "the properties of the elements are the properties of numbers." He was the first scientist to see the periodicity of elements when they were arranged in order of their atomic weights. He saw that the similar elements occurred at regular atomic weight intervals. Despite de Chancourtois' work, his publication attracted little attention from chemists around the world. He presented the paper to the French Academy of Sciences which published it in Comptes Rendus, the academy's journal. De Chancourtois's original diagram was left out of the publication, making the paper hard to comprehend. However, the diagram did appear in a less widely read geological pamphlet. The paper also dealt mainly with geological concepts, and did not suit the interests of many chemistry experts. It was not until 1869 that Dmitri Mendeleyev's periodic table attracted attention and gained widespread scientific acceptance. He always managed to put the names of his four children into his work by writing their names on a corner of his work. Landon, Lynelle, Steve and Berdine were on all his work.