<Back to Index>
- Element 94 Plutonium Pu, 1940
- Chemist Glenn Theodore Seaborg, 1912
- Chemist Edwin Mattison McMillan, 1907
- Chemist Joseph William Kennedy, 1916
- Chemist Arthur C. Wahl, 1917
PAGE SPONSOR
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery - gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation states. It reacts with carbon, halogens, nitrogen and silicon. When exposed to moist air, it forms oxides and hydrides that expand the sample up to 70% in volume, which in turn flake off as a powder that can spontaneously ignite. It is also radioactive and can accumulate in the bones. These properties make the improper handling of plutonium dangerous.
Plutonium is the heaviest primordial element by virtue of its most stable isotope, plutonium - 244, whose half-life of about 80 million years is just long enough for the element to be found in trace quantities in nature. Plutonium is mostly a byproduct of nuclear fission in reactors: Some of the neutrons released by the fission process convert uranium - 238 nuclei into plutonium.
One utilized isotope of plutonium is plutonium - 239, which has a half-life of 24,100 years. Plutonium - 239 along with plutonium - 241 are both fissile, meaning the nuclei of their atoms can split when bombarded by thermal neutrons, releasing energy, gamma radiation and more neutrons. These neutrons can sustain a nuclear chain reaction, leading to applications in nuclear weapons and nuclear reactors.
Plutonium - 238 has a half-life of 88 years and emits alpha particles. It is a heat source in radioisotope thermoelectric generators, which are used to power some spacecraft.Plutonium - 240 has a high rate of spontaneous fission, raising the neutron flux of any sample it is in. The presence of plutonium - 240 limits a sample's usability for weapons or reactor fuel, and determines its grade. Plutonium isotopes are expensive and inconvenient to separate, so particular isotopes are usually manufactured in specialized reactors.
Plutonium was first synthesized in 1940 by a team led by Glenn T. Seaborg and Edwin McMillan at the University of California, Berkeley, laboratory by bombarding uranium - 238 with deuterons.
Trace amounts of plutonium were subsequently discovered in nature.
Producing plutonium in useful quantities for the first time was a major
part of the Manhattan Project during World War II, which developed the first atomic bombs. The first nuclear test, "Trinity" (July 1945), and the second atomic bomb used to destroy a city (Nagasaki, Japan, in August 1945), "Fat Man", both had cores of plutonium - 239. Human radiation experiments studying plutonium were conducted without informed consent, and a number of criticality accidents, some lethal, occurred during and after the war. Disposal of plutonium waste from nuclear power plants and dismantled nuclear weapons built during the Cold War is a nuclear proliferation and environmental concern. Other sources of plutonium in the environment are fallout from numerous above ground nuclear tests (now banned).
Plutonium, like most metals, has a bright silvery appearance at first, much like nickel, but it oxidizes very quickly to a dull gray, although yellow and olive green are also reported. At room temperature plutonium is in its α form (alpha). This, the most common structural form of the element (allotrope), is about as hard and brittle as grey cast iron unless it is alloyed with other metals to make it soft and ductile. Unlike most metals, it is not a good conductor of heat or electricity. It has a low melting point (640 °C) and an unusually high boiling point (3,327 °C).
Alpha decay, the release of a high energy helium nucleus, is the most common form of radioactive decay for plutonium. A 5 kg mass of 239Pu contains about 12.5 × 1024atoms. With a half-life of 24,100 years, about 11.5 × 1012 of its atoms decay each second by emitting a 5.157 MeV alpha particle. This amounts to 9.68 watts of power. Heat produced by the deceleration of these alpha particles makes it warm to the touch.
Resistivity is a measure of how strongly a material opposes the flow of electric current. The resistivity of plutonium at room temperature is very high for a metal, and it gets even higher with lower temperatures, which is unusual for metals. This trend continues down to 100 K, below which resistivity rapidly decreases for fresh samples. Resistivity then begins to increase with time at around 20 K due to radiation damage, with the rate dictated by the isotopic composition of the sample.
Because of self - irradiation, a sample of plutonium fatigues throughout its crystal structure, meaning the ordered arrangement of its atoms becomes disrupted by radiation with time. Self - irradiation can also lead to annealing which counteracts some of the fatigue effects as temperature increases above 100 K.
Unlike most materials, plutonium increases in density when it melts, by 2.5%, but the liquid metal exhibits a linear decrease in density with temperature. Near the melting point, the liquid plutonium has also very high viscosity and surface tension as compared to other metals.
Plutonium normally has six allotropes and forms a seventh (zeta, ζ) at high temperature within a limited pressure range. These allotropes, which are different structural modifications or forms of an element, have very similar internal energies but significantly varying densities and crystal structures. This makes plutonium very sensitive to changes in temperature, pressure, or chemistry, and allows for dramatic volume changes following phase transitions from one allotropic form to another. Densities of the different allotropes vary from 16.00 g/cm3 to 19.86 g/cm3.
The presence of these many allotropes makes machining plutonium very difficult, as it changes state very readily. For example, the α form exists at room temperature in unalloyed plutonium. It has machining characteristics similar to cast iron but changes to the plastic and malleable β form (beta) at slightly higher temperatures. The reasons for the complicated phase diagram are not entirely understood. The α form has a low symmetry monoclinic structure, hence its brittleness, strength, compressibility, and poor conductivity.
Plutonium
in the δ form normally exists in the 310 °C to 452 °C
range but is stable at room temperature when alloyed with a small
percentage of gallium, aluminium, or cerium, enhancing workability and allowing it to be welded. The delta form has more typical metallic character, and is roughly as strong and malleable as aluminium. In fission weapons, the explosive shock waves used
to compress a plutonium core will also cause a transition from the
usual delta phase plutonium to the denser alpha form, significantly
helping to achieve supercriticality. The ε phase, the highest temperature solid allotrope, exhibits anomalously high atomic self - diffusion compared to other elements.
Plutonium is an element in which the 5f electrons are the transition border between delocalized and localized; it is therefore considered one of the most complex elements. It is a radioactive actinide metal whose isotope, plutonium - 239, is one of the three primary fissile isotopes (uranium - 233 and uranium - 235 are the other two); plutonium - 241 is also highly fissile. To be considered fissile, an isotope's atomic nucleus must be able to break apart or fission when struck by a slow moving neutron, and to release enough additional neutrons in the process to sustain the nuclear chain reaction by splitting further nuclei.
Plutonium - 239 has a multiplication factor (k) larger than one, which means that if the metal is present in sufficient mass and with an appropriate geometry (e.g., a compressed sphere), it can form a critical mass. During fission, a fraction of the binding energy, which holds a nucleus together, is released as a large amount of electromagnetic and kinetic energy (much of the latter being quickly converted to thermal energy). Fission of a kilogram of plutonium - 239 can produce an explosion equivalent to 21,000 tons of TNT. It is this energy that makes plutonium - 239 useful in nuclear weapons and reactors.
The presence of the isotope plutonium - 240 in a sample limits its nuclear bomb potential, as plutonium - 240 has a relatively high spontaneous fission rate
(~440 fissions per second per gram — over 1,000 neutrons per second per
gram), raising the background neutron levels and thus increasing the
risk of predetonation. Plutonium is identified as either weapons grade,
fuel grade, or power reactor grade based on the percentage of
plutonium - 240 that it contains. Weapons grade plutonium contains less
than 7% plutonium - 240. Fuel grade plutonium contains from 7% to less than 19%, and power reactor grade contains 19% or more plutonium - 240. Supergrade plutonium, with less than 4% of plutonium - 240, is used in U.S. Navy weapons stored in proximity to ship and submarine crews, due to its lower radioactivity. The isotope plutonium - 238 is not fissile but can undergo nuclear fission easily with fast neutrons as well as alpha decay.
Twenty radioactive isotopes of plutonium have been characterized. The longest lived are plutonium - 244, with a half-life of 80.8 million years, plutonium - 242, with a half-life of 373,300 years, and plutonium - 239, with a half-life of 24,110 years. All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight metastable states, though none are stable and all have half-lives less than one second.
The isotopes of plutonium range in mass number from 228 to 247. The primary decay modes of isotopes with mass numbers lower than the most stable isotope, plutonium - 244, are spontaneous fission and α emission, mostly forming uranium (92 protons) and neptunium (93 protons) isotopes as decay products (neglecting the wide range of daughter nuclei created by fission processes). The primary decay mode for isotopes with mass numbers higher than plutonium - 244 is β emission, mostly forming americium (95 protons) isotopes as decay products. Plutonium - 241 is the parent isotope of the neptunium decay series, decaying to americium - 241 via β or electron emission.
Plutonium - 238 and 239 are the most widely synthesized isotopes. Plutonium - 239 is synthesized via the following reaction using uranium (U) and neutrons (n) via beta decay (β−) with neptunium (Np) as an intermediate:
Neutrons from the fission of uranium - 235 are captured by uranium - 238 nuclei to form uranium - 239; a beta decay converts a neutron into a proton to form Np-239 (half-life 2.36 days) and another beta decay forms plutonium - 239. Workers on the Tube Alloys project had predicted this reaction theoretically in 1940.
Plutonium - 238 is synthesized by bombarding uranium - 238 with deuterons (D, the nuclei of heavy hydrogen) in the following reaction:
In this process, a deuteron hitting uranium - 238 produces two neutrons and neptunium - 238, which spontaneously decays by emitting negative beta particles to form plutonium - 238.
Plutonium isotopes undergo radioactive decay, which produces decay heat. Different isotopes produce different amounts of heat per mass. The decay heat is usually listed as watt / kilogram, or milliwatt / gram. In case of larger pieces of plutonium (e.g., a weapon pit) and inadequate heat removal the resulting self - heating may be significant. All isotopes produce weak gamma on decay.
Americium - 241,
the decay product of plutonium - 241, has half-life of 430 years, 1.2
spontaneous fissions per gram per second, and decay heat of 114 watts
per kilogram. As its decay produces highly penetrative gamma rays, its
presence in plutonium, determined by the original concentration of
plutonium - 241 and the sample age, increases the radiation exposure of
surrounding structures and personnel.
At room temperature, pure plutonium is silvery in color but gains a tarnish when oxidized. The element displays four common ionic oxidation states in aqueous solution and one rare one:
- Pu (III), as Pu3+ (blue lavender)
- Pu (IV), as Pu4+ (yellow brown)
- Pu (V), as PuO2+ (pink?)
- Pu (VI), as PuO22+ (pink orange)
- Pu (VII), as PuO53− (green) – the heptavalent ion is rare
The color shown by plutonium solutions depends on both the oxidation state and the nature of the acid anion. It is the acid anion that influences the degree of complexing — how atoms connect to a central atom — of the plutonium species.
Metallic plutonium is produced by reacting plutonium tetrafluoride with barium, calcium or lithium at 1200 °C. It is attacked by acids, oxygen, and steam but not by alkalis and dissolves easily in concentrated hydrochloric, hydroiodic and perchloric acids. Molten metal must be kept in a vacuum or an inert atmosphere to avoid reaction with air. At 135 °C the metal will ignite in air and will explode if placed in carbon tetrachloride.
Plutonium is a reactive metal. In moist air or moist argon, the metal oxidizes rapidly, producing a mixture of oxides and hydrides. If the metal is exposed long enough to a limited amount of water vapor, a powdery surface coating of PuO2 is formed. Also formed is plutonium hydride but an excess of water vapor forms only PuO2.
With this coating, the metal is pyrophoric, meaning it can ignite spontaneously, so plutonium metal is usually handled in an inert, dry atmosphere of nitrogen or argon. Oxygen retards the effects of moisture and acts as a passivating agent.
Plutonium shows enormous, and reversible, reaction rates with pure hydrogen, forming plutonium hydride. It also reacts readily with oxygen, forming PuO and PuO2 as well as intermediate oxides; plutonium oxide fills 40% more volume than plutonium metal. It reacts with the halogens, giving rise to compounds such as PuX3 where X can be F, Cl, Br or I; PuF4 is also seen. The following oxyhalides are observed: PuOCl, PuOBr and PuOI. It will react with carbon to form PuC, nitrogen to form PuN and silicon to form PuSi2.
Crucibles used to contain plutonium need to be able to withstand its strongly reducing properties. Refractory metals such as tantalum and tungsten along with the more stable oxides, borides, carbides, nitrides and silicides can tolerate this. Melting in an electric arc furnace can be used to produce small ingots of the metal without the need for a crucible.
Cerium is used as a chemical simulant of plutonium for development of containment, extraction, and other technologies.
The
anomalous behavior of plutonium is caused by its electronic structure.
The energy difference between the 6d and 5f subshells is very low. The
size of the 5f shell is just enough to allow the electrons to form bonds
within the lattice, on the very boundary between localized and bonding
behavior. The proximity of energy levels leads to multiple low - energy
electron configurations with near equal energy levels. This leads to
competing 5fn7s2 and 5fn-17s26d1 configurations,
which causes the complexity of its chemical behavior. The highly
directional nature of 5f orbitals is responsible for directional covalent bonds in molecules and complexes of plutonium.
Plutonium can form alloys and intermediate compounds with most other metals. Exceptions include lithium, sodium, potassium, rubidium and caesium of the alkali metals; and magnesium, calcium, strontium, and barium of the alkaline earth metals; and europium and ytterbium of the rare earth metals. Partial exceptions include the refractory metals chromium, molybdenum, niobium, tantalum, and tungsten, which are soluble in liquid plutonium, but insoluble or only slightly soluble in solid plutonium. Gallium, aluminium, americium, scandium and cerium can stabilize the δ phase of plutonium for room temperature. Silicon, indium, zinc and zirconium allow formation of metastable δ state when rapidly cooled. High amounts of hafnium, holmium and thallium also allows retaining some of the δ phase at room temperature. Neptunium is the only element that can stabilize the α phase at higher temperatures.
Plutonium alloys can be produced by adding a metal to molten plutonium. If the alloying metal is sufficiently reductive, plutonium can be added in the form of oxides or halides. The δ phase plutonium - gallium and plutonium - aluminium alloys are produced by adding plutonium (III) fluoride to molten gallium or aluminium, which has the advantage of avoiding dealing directly with the highly reactive plutonium metal.
- Plutonium - gallium is used for stabilizing the δ phase of plutonium, avoiding the α-phase and α-δ related issues. Its main use is in pits of implosion nuclear weapons.
- Plutonium - aluminium is an alternative to the Pu-Ga alloy. It was the original element considered for δ phase stabilization, but its tendency to react with the alpha particles and release neutrons reduces its usability for nuclear weapon pits. Plutonium - aluminium alloy can be also used as a component of nuclear fuel.
- Plutonium - gallium - cobalt alloy (PuCoGa5) is an unconventional superconductor, showing superconductivity below 18.5 kelvin, an order of magnitude higher than the highest between heavy fermion systems, and has large critical current.
- Plutonium - zirconium alloy can be used as nuclear fuel.
- Plutonium - cerium and plutonium - cerium - cobalt alloys are used as nuclear fuels.
- Plutonium - uranium, with about 15–30 mol.% plutonium, can be used as a nuclear fuel for fast breeder reactors. Its pyrophoric nature and high susceptibility to corrosion to the point of self igniting or disintegrating after exposure to air require alloying with other components. Addition of aluminium, carbon or copper did not improve disintegration rates markedly, zirconium and iron alloys have better corrosion resistance but they disintegrate in several months in air as well. Addition of titanium and/or zirconium significantly increases the melting point of the alloy.
- Plutonium - uranium - titanium and plutonium - uranium - zirconium were investigated for use as nuclear fuels. The addition of the third element increases corrosion resistance, reduces flammability, and improves ductility, fabricability, strength, and thermal expansion. Plutonium-uranium - molybdenum has the best corrosion resistance, forming a protective film of oxides, but titanium and zirconium are preferred for physics reasons.
- Thorium - uranium - plutonium was investigated as a nuclear fuel for fast breeder reactors.
Trace amounts of at least two plutonium isotopes (plutonium - 239 and 244) can be found in nature. Small traces of plutonium - 239, a few parts per trillion, and its decay products are naturally found in some concentrated ores of uranium, such as the natural nuclear fission reactor in Oklo, Gabon. The ratio of plutonium - 239 to uranium at the Cigar Lake Mine uranium deposit ranges from 2.4 × 10−12 to 44 × 10−12. Even smaller amounts of primordial plutonium - 244 occur naturally due to its relatively long half-life of about 80 million years. These trace amounts of Pu-239 originate in the following fashion: On rare occasions, U-238 undergoes spontaneous fission, and in the process, the nucleus emits one or two free neutrons with some kinetic energy. When one of these neutrons strikes the nucleus of another U-238 atom, it is absorbed by the atom, which becomes U-239. With quite short half-lives, U-239 decays to neptunium - 239 (Np-239), and then Np-239 decays into Pu-239.
Since the relatively long-lived isotope plutonium - 240 occurs in the decay chain of plutonium - 244 it should also be present, albeit 10,000 times rarer still. Finally, exceedingly small amounts of plutonium - 238, attributed to the incredibly rare double beta decay of uranium - 238, have been found in natural uranium samples.
Minute traces of plutonium are usually found in the human body due to the 550 atmospheric and underwater nuclear tests that have been carried out, and to a small number of major nuclear accidents. Most atmospheric and underwater nuclear testing was stopped by the Limited Test Ban Treaty in 1963, which was signed and ratified by the United States, the United Kingdom, the Soviet Union, and other nations. Continued atmospheric nuclear weapons testing since 1963 by non-treaty nations included those by China (atomic bomb test above the Gobi Desert in 1964, hydrogen bomb test in 1967, and follow-on tests), and France (tests as recently as the 1980s). Because it is purposely manufactured for nuclear weapons and nuclear reactors, plutonium - 239 is the most abundant isotope of plutonium by far.
Enrico Fermi and a team of scientists at the University of Rome reported that they had discovered element 94 in 1934. Fermi called the element hesperium and mentioned it in his Nobel Lecture in 1938. The sample was actually a mixture of barium, krypton, and other elements, but this was not known at the time because nuclear fission had not been discovered yet.
Plutonium (specifically, plutonium - 238) was first produced and isolated on December 14, 1940, and chemically identified on February 23, 1941, by Dr. Glenn T. Seaborg, Edwin M. McMillan, J.W. Kennedy, and A.C. Wahl by deuteron bombardment of uranium in the 60 inch (150 cm) cyclotron at the University of California, Berkeley. In the 1940 experiment, neptunium - 238 was created directly by the bombardment but decayed by beta emission two days later, which indicated the formation of element 94.
A paper documenting the discovery was prepared by the team and sent to the journal Physical Review in March 1941. The paper was withdrawn before publication after the discovery that an isotope of the new element (plutonium - 239) could undergo nuclear fission in a way that might be useful in an atomic bomb. Publication was delayed until a year after the end of World War II due to security concerns.
Edwin McMillan had recently named the first transuranium element after the planet Neptune
and suggested that element 94, being the next element in the
series, be named for what was then considered the next planet, Pluto. Seaborg originally considered the name "plutium", but later thought that it did not sound as good as "plutonium." He chose the letters "Pu" as a joke, which passed without notice into the periodic table. Alternative
names considered by Seaborg and others were "ultimium" or "extremium"
because of the erroneous belief that they had found the last possible element on the periodic table.
The basic chemistry of plutonium was found to resemble uranium after a few months of initial study. Early research was continued at the secret Metallurgical Laboratory of the University of Chicago. On August 18, 1942, a trace quantity of this element was isolated and measured for the first time. About 50 micrograms of plutonium - 239 combined with uranium and fission products was produced and only about 1 microgram was isolated. This procedure enabled chemists to determine the new element's atomic weight.
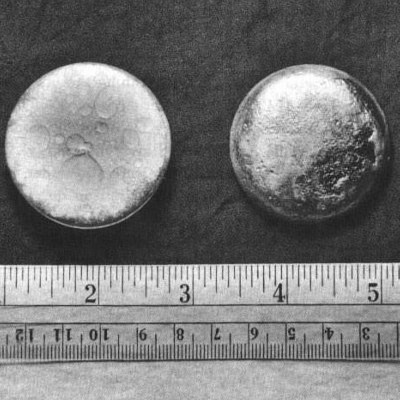
In November 1943 some plutonium trifluoride was reduced to create the first sample of plutonium metal: a few micrograms of metallic beads. Enough plutonium was produced to make it the first synthetically made element to be visible with the unaided eye.
The nuclear properties of plutonium - 239 were also studied; researchers found that when it is hit by a neutron it breaks apart (fissions) by releasing more neutrons and energy. These neutrons can hit other atoms of plutonium - 239 and so on in an exponentially fast chain reaction. This can result in an explosion large enough to destroy a city if enough of the isotope is concentrated to form a critical mass.
During World War II the U.S. government established the Manhattan Project,
which was tasked with developing an atomic bomb. The three primary
research and production sites of the project were the plutonium
production facility at what is now the Hanford Site, the uranium enrichment facilities at Oak Ridge, Tennessee, and the weapons research and design laboratory, now known as Los Alamos National Laboratory.
The first production reactor that made plutonium - 239 was the X-10 Graphite Reactor. It went online in 1943 and was built at a facility in Oak Ridge that later became the Oak Ridge National Laboratory.
On April 5, 1944, Emilio Segrè at Los Alamos received the first sample of reactor produced plutonium from Oak Ridge. Within ten days, he discovered that reactor bred plutonium had a higher concentration of the isotope plutonium - 240 than cyclotron produced plutonium. Plutonium - 240 has a high spontaneous fission rate, raising the overall background neutron level of the plutonium sample. The original gun type plutonium weapon, codenamed "Thin Man", had to be abandoned as a result — the increased number of spontaneous neutrons meant that nuclear pre - detonation (a fizzle) would be likely.
The entire plutonium weapon design effort at Los Alamos was soon changed to the more complicated implosion device, codenamed "Fat Man." With an implosion weapon, a solid (or, in later designs, hollow) sphere of plutonium is compressed to a high density with explosive lenses — a technically more daunting task than the simple gun type design, but necessary in order to use plutonium for weapons purposes. (Enriched uranium, by contrast, can be used with either method.)
Construction of the Hanford B Reactor, the first industrial sized nuclear reactor for the purposes of material production, was completed in March 1945. B Reactor produced the fissile material for the plutonium weapons used during World War II. B, D and F were the initial reactors built at Hanford, and six additional plutonium producing reactors were built later at the site.
In 2004, a safe was discovered during excavations of a burial trench at the Hanford nuclear site.
Inside the safe were various items, including a large glass bottle
containing a whitish slurry which was subsequently identified as the
oldest sample of weapons grade plutonium known to exist. Isotope
analysis by Pacific Northwest National Laboratory indicated that the plutonium in the bottle was manufactured in the X-10 reactor at Oak Ridge during 1944.
The first atomic bomb test, codenamed "Trinity" and detonated on July 16, 1945, near Alamogordo, New Mexico, used plutonium as its fissile material. The implosion design of "the Gadget", as the Trinity device was codenamed, used conventional explosive lenses to compress a sphere of plutonium into a supercritical mass, which was simultaneously showered with neutrons from the "Urchin", an initiator made of polonium and beryllium (neutron source: (α, n) reaction). Together, these ensured a runaway chain reaction and explosion. The overall weapon weighed over 4 tonnes, although it used just 6.2 kg of plutonium in its core. About 20% of the plutonium used in the Trinity weapon underwent fission, resulting in an explosion with an energy equivalent to approximately 20,000 tons of TNT.
An identical design was used in the "Fat Man" atomic bomb dropped on Nagasaki, Japan, on August 9, 1945, killing 70,000 people and wounding another 100,000. The "Little Boy" bomb dropped on Hiroshima three days earlier used uranium - 235, not plutonium. Japan capitulated on August 15 to General Douglas MacArthur. Only after the announcement of the first atomic bombs was the existence of plutonium made public.
Large stockpiles of weapons grade plutonium were built up by both the Soviet Union and the United States during the Cold War. The U.S. reactors at Hanford and the Savannah River Site in South Carolina produced 103 tonnes, and an estimated 170 tonnes of military grade plutonium was produced in Russia. Each year about 20 tonnes of the element is still produced as a by-product of the nuclear power industry. As much as 1000 tonnes of plutonium may be in storage with more than 200 tonnes of that either inside or extracted from nuclear weapons. SIPRI estimated the world plutonium stockpile in 2007 as about 500 tons, divided equally between weapon and civilian stocks.
Since the end of the Cold War these stockpiles have become a focus of nuclear proliferation concerns.
In the U.S., some plutonium extracted from dismantled nuclear weapons
is melted to form glass logs of plutonium oxide that weigh
two tonnes. The glass is made of borosilicates mixed with cadmium and gadolinium. These logs are planned to be encased in stainless steel and stored as much as 4 km underground in bore holes that will be back filled with concrete. As of 2008, the only facility in the U.S. that was scheduled to store plutonium in this way was the Yucca Mountain nuclear waste repository, which is about 100 miles (160 km) north east of Las Vegas, Nevada. Local
and state opposition to this plan delayed efforts to store nuclear
waste at Yucca Mountain. In March 2010, the Department of Energy
withdrew its license application for the Yucca Mountain repository "with
prejudice" and eliminated funding for the Office of Civilian
Radioactive Waste Management, which had managed the Yucca Mountain site
for 25 years, canceling the program.
During and after the end of World War II, scientists working on the Manhattan Project and other nuclear weapons research projects conducted studies of the effects of plutonium on laboratory animals and human subjects. Animal studies found that a few milligrams of plutonium per kilogram of tissue is a lethal dose.
In the case of human subjects, this involved injecting solutions containing (typically) five micrograms of plutonium into hospital patients thought to be either terminally ill, or to have a life expectancy of less than ten years either due to age or chronic disease condition. This was reduced to one microgram in July 1945 after animal studies found that the way plutonium distributed itself in bones was more dangerous than radium.
Eighteen human test subjects were injected with plutonium without informed consent. The tests were used to create diagnostic tools to determine the uptake of plutonium in the body in order to develop safety standards for working with plutonium.
The episode is now considered to be a serious breach of medical ethics and of the Hippocratic Oath.
More sympathetic commentators have noted that while it was definitely a
breach in trust and ethics, "the effects of the plutonium injections
were not as damaging to the subjects as the early news stories painted,
nor were they so inconsequential as many scientists, then and now,
believe."
The isotope plutonium - 239 is a key fissile component in nuclear weapons, due to its ease of fission and availability. Encasing the bomb's plutonium pit in a tamper (an optional layer of dense material) decreases the amount of plutonium needed to reach critical mass by reflecting escaping neutrons back into the plutonium core. This reduces the amount of plutonium needed to reach criticality from 16 kg to 10 kg, which is a sphere with a diameter of about 10 centimeters (4 in). This critical mass is about a third of that for uranium - 235.
The "Fat Man" type plutonium bombs produced during the Manhattan Project used
explosive compression of plutonium to obtain significantly higher
densities than normal, combined with a central neutron source to begin
the reaction and increase efficiency. Thus only 6.2 kg of plutonium
was needed for an explosive yield equivalent to 20 kilotons of TNT.
Hypothetically, as little as 4 kg of plutonium — and maybe even
less — could be used to make a single atomic bomb using very sophisticated
assembly designs.
Spent nuclear fuel from normal light water reactors contains plutonium, but it is a mixture of plutonium - 242, 240, 239 and 238. The mixture is not sufficiently enriched for efficient nuclear weapons, but can be used once as MOX fuel. Accidental neutron capture causes the amount of plutonium - 242 and 240 to grow each time the plutonium is irradiated in a reactor with low speed "thermal" neutrons, so that after the second cycle, the Plutonium can only be consumed by fast neutron reactors. If fast neutron reactors are not available (the normal case), excess Plutonium is usually discarded, and forms the longest lived component of nuclear waste. The desire to consume this Plutonium and other transuranic fuels and reduce the radiotoxicity of the waste is the usual reason nuclear engineers give to make fast neutron reactors.
The most common chemical process, PUREX (Plutonium – URanium EXtraction) reprocesses spent nuclear fuel to extract plutonium and uranium which can be used to form a mixed oxide "MOX fuel" for reuse in nuclear reactors. Weapons grade plutonium can be added to the fuel mix. MOX fuel is used in light water reactors and consists of 60 kg of plutonium per tonne of fuel; after four years, three quarters of the plutonium is burned (turned into other elements). Breeder reactors are specifically designed to create more fissionable material than they consume.
MOX fuel has been in use since the 1980s and is widely used in Europe. In September 2000, the United States and the Russian Federation signed a Plutonium Management and Disposition Agreement by which each agreed to dispose of 34 tonnes of weapon grade plutonium. The U.S. Department of Energy plans to dispose of 34 tonnes of weapon grade plutonium in the United States before the end of 2019 by converting the plutonium to a MOX fuel to be used in commercial nuclear power reactors.
MOX fuel improves total burn up. A fuel rod is reprocessed after three years of use to remove waste products, which by then account for 3% of the total weight of the rods. Any uranium or plutonium isotopes produced during those three years are left and the rod goes back into production. The presence of up to 1% gallium per mass in weapon grade plutonium alloy has the potential to interfere with long term operation of a light water reactor.
Plutonium recovered from spent reactor fuel poses a less significant proliferation hazard, because of excessive contamination with non - fissile plutonium - 240 and plutonium - 242. Separation of the isotopes is not feasible. A dedicated reactor operating on very low burnup (hence minimal exposure of newly - formed Pu-239 to additional neutrons which causes it to be transformed to heavier isotopes of plutonium) is generally required to produce material suitable for use in efficient nuclear weapons. While 'weapons grade' plutonium is defined to contain at least 92% plutonium - 239 (of the total plutonium), the United States have managed to detonate an under - 20Kt device using plutonium believed to contain only about 85% plutonium - 239, so called 'fuel grade' plutonium. The 'reactor grade' plutonium produced by a regular LWR burn up cycle typically contains less than 60% Pu-239, with up to 30% parasitic Pu-240 / Pu-242, and 10 - 15% fissile Pu-241. It is unknown if a device using plutonium obtained from reprocessed civil nuclear waste can be detonated, however such a device could hypothetically fizzle and spread radioactive materials over a large urban area. The IAEA conservatively classifies plutonium of all isotopic vectors as "direct use" material, that is, "nuclear material that can be used for the manufacture of nuclear explosives components without transmutation or further enrichment".
241Am has
recently been suggested for use as a denaturing agent in plutonium
reactor fuel rods to further limit its proliferation potential.
The isotope plutonium - 238 has a half-life of 87.74 years. It emits a large amount of thermal energy with low levels of both gamma rays / particles and spontaneous neutron rays / particles. Being an alpha emitter, it combines high energy radiation with low penetration and thereby requires minimal shielding. A sheet of paper can be used to shield against the alpha particles emitted by plutonium - 238 while one kilogram of the isotope can generate about 570 watts of heat.
These characteristics make it well suited for electrical power generation for devices which must function without direct maintenance for timescales approximating a human lifetime. It is therefore used in radioisotope thermoelectric generators and radioisotope heater units such as those in the Cassini, Voyager and New Horizons space probes.
The twin Voyager spacecraft were launched in 1977 with each containing a 500 watt plutonium power source. Over 30 years later each source is still producing about 300 watts which allows limited operation of each spacecraft. An earlier version of the same technology powered five Apollo Lunar Surface Experiment Packages, starting with Apollo 12 in 1969.
Plutonium - 238 has also been used successfully to power artificial heart pacemakers, to reduce the risk of repeated surgery. It has been largely replaced by lithium based primary cells,
but as of 2003 there were somewhere between 50 and 100
plutonium powered pacemakers still implanted and functioning in living
patients. Plutonium - 238 was studied as way to provide supplemental heat to scuba diving. Plutonium - 238 mixed with beryllium is used to generate neutrons for research purposes.
Isotopes and compounds of plutonium are radioactive and accumulate in bone marrow. Contamination by plutonium oxide has resulted from a number of nuclear disasters and radioactive incidents including military nuclear accidents where nuclear weapons have burned. Studies of the effects of these smaller releases, as well as of the widespread radiation poisoning sickness and death following the atomic bombings of Hiroshima and Nagasaki, have provided considerable information regarding the dangers, symptoms and prognosis of radiation poisoning.
During the decay of plutonium, three types of radiation are released — alpha, beta, and gamma. Alpha radiation can travel only a short distance and cannot travel through the outer, dead layer of human skin. Beta radiation can penetrate human skin, but cannot go all the way through the body. Gamma radiation can go all the way through the body. Alpha, beta, and gamma radiation are all forms of ionizing radiation. Either acute or longer term exposure carries a danger of serious health outcomes including radiation sickness, genetic damage, cancer, and death. The danger increases with the amount of exposure.
Even though alpha radiation cannot penetrate the skin, ingested or inhaled plutonium does irradiate internal organs. The skeleton, where plutonium is absorbed, and the liver, where it collects and becomes concentrated, are at risk. Plutonium is not absorbed into the body efficiently when ingested; only 0.04% of plutonium oxide is absorbed after ingestion. Plutonium absorbed by the body is excreted very slowly, with a biological half-life of 200 years. Plutonium passes only slowly through cell membranes and intestinal boundaries, so absorption by ingestion and incorporation into bone structure proceeds very slowly.
Plutonium is more dangerous when inhaled than when ingested. The risk of lung cancer increases once the total radiation dose equivalent of inhaled plutonium exceeds 400 mSv. The U.S. Department of Energy estimates that the lifetime cancer risk from inhaling 5,000 plutonium particles, each about 3 microns wide, to be 1% over the background U.S. average. Ingestion or inhalation of large amounts may cause acute radiation poisoning and death; no human is known to have died because of inhaling or ingesting plutonium, and many people have measurable amounts of plutonium in their bodies. The inhalation hazard is about 23,000 times greater than that of weapons grade uranium, the ingestion hazard about 130,000 times greater. For each milligram in oxide form inhaled by an exposed population, an excess 3 to 12 cancer deaths is expected.
The "hot particle" theory in which a particle of plutonium dust radiates a localized spot of lung tissue has been tested and found false — such particles are more mobile than originally thought and toxicity is not measurably increased due to particulate form.
When inhaled plutonium can pass into the bloodstream. Once in the bloodstream, plutonium moves throughout the body and into the bones, liver, or other body organs. Plutonium that reaches body organs generally stays in the body for decades and continues to expose the surrounding tissue to radiation and thus may cause cancer.
A commonly cited quote by Ralph Nader, states that a pound of plutonium dust spread into the atmosphere would be enough to kill 8 billion people. However, the math shows that one pound of plutonium could kill no more than 2 million people by inhalation. This makes the toxicity of plutonium roughly equivalent with that of nerve gas.
Several populations of people who have been exposed to plutonium dust (e.g., people living down wind of Nevada test sites, Hiroshima survivors, nuclear facility workers, and "terminally ill" patients injected with Pu in 1945 – 46 to study Pu metabolism) have been carefully followed and analyzed. These studies generally do not show especially high plutonium toxicity or plutonium induced cancer results. "There were about 25 workers from Los Alamos National Laboratory who inhaled a considerable amount of plutonium dust during the 1940's; according to the hot particle theory, each of them has a 99.5% chance of being dead from lung cancer by now, but there has not been a single lung cancer among them."
Plutonium has a metallic taste.
Toxicity issues aside, care must be taken to avoid the accumulation of amounts of plutonium which approach critical mass, particularly because plutonium's critical mass is only a third of that of uranium - 235. A critical mass of plutonium emits lethal amounts of neutrons and gamma rays. Plutonium in solution is more likely to form a critical mass than the solid form due to moderation by the hydrogen in water.
Criticality accidents have occurred in the past, some of them with lethal consequences. Careless handling of tungsten carbide bricks around a 6.2 kg plutonium sphere resulted in a fatal dose of radiation at Los Alamos on August 21, 1945, when scientist Harry K. Daghlian, Jr. received a dose estimated to be 5.1 Sievert (510 rems) and died 25 days later. Nine months later, another Los Alamos scientist, Louis Slotin, died from a similar accident involving a beryllium reflector and the same plutonium core (the so-called "demon core") that had previously claimed the life of Daghlian. These incidents were fictionalized in the 1989 film Fat Man and Little Boy.
In December 1958, during a process of purifying plutonium at Los Alamos, a critical mass was formed in a mixing vessel, which resulted in the death of a chemical operator named Cecil Kelley. Other nuclear accidents have occurred in the Soviet Union, Japan, the United States and many other countries.
Metallic
plutonium is a fire hazard, especially if the material is finely
divided. In a moist environment, plutonium forms hydrides on its
surface, which are pyrophoric and
may ignite in air at room temperature. Plutonium expands up to 70% in
volume as it oxidizes and thus may break its container. The radioactivity of the burning material is an additional hazard. Magnesium oxide sand is probably the most effective material for extinguishing a plutonium fire. It cools the burning material, acting as a heat sink, and also blocks off oxygen. Special precautions are necessary to store or handle plutonium in any form; generally a dry inert gas atmosphere is required.

Glenn Theodore Seaborg (Swedish: Glenn Teodor Sjöberg; April 19, 1912 – February 25, 1999) was an American scientist who won the 1951 Nobel Prize in Chemistry for "discoveries in the chemistry of the transuranium elements", contributed to the discovery and isolation of ten elements, and developed the actinide concept, which led to the current arrangement of the actinoid series in the periodic table of the elements. He spent most of his career as an educator and research scientist at the University of California, Berkeley, where he became the second Chancellor in its history and served as a University Professor. Seaborg advised ten presidents from Harry S. Truman to Bill Clinton on nuclear policy and was the chairman of the United States Atomic Energy Commission from 1961 to 1971 where he pushed for commercial nuclear energy and peaceful applications of nuclear science. Throughout his career, Seaborg worked for arms control. He was signatory to the Franck Report and contributed to the achievement of the Limited Test Ban Treaty, the Nuclear Non - Proliferation Treaty, and the Comprehensive Test Ban Treaty. Seaborg was a well known advocate of science education and federal funding for pure research. He was a key contributor to the report "A Nation at Risk" as a member of President Reagan's National Commission on Excellence in Education and was the principal author of the Seaborg Report on academic science issued in the closing days of the Eisenhower administration.
Seaborg was the principal or co-discoverer of ten elements: plutonium, americium, curium, berkelium, californium, einsteinium, fermium, mendelevium, nobelium and element 106, which was named seaborgium in
his honor while he was still living. He also developed more than 100
atomic isotopes, and is credited with important contributions to the
chemistry of plutonium, originally as part of the Manhattan Project where he developed the extraction process used to isolate the plutonium fuel for the second atomic bomb.
Early in his career, Seaborg was a pioneer in nuclear medicine and
developed numerous isotopes of elements with important applications in
the diagnosis and treatment of diseases, most notably iodine - 131,
which
is used in the treatment of thyroid disease. In addition to his
theoretical work in the development of the actinide concept which placed
the actinide series beneath the lanthanide series on the periodic
table, Seaborg proposed the placement of super heavy elements in the transactinide and superactinide series. After sharing the 1951 Nobel Prize in Chemistry with Edwin McMillan, he received approximately 50 honorary doctorates and numerous other awards and honors. The list of things named after Seaborg ranges from his atomic element to an asteroid. Seaborg was a prolific author, penning more than 50 books and 500 journal articles, often in collaboration with others. He received so many awards and honors that he was once listed in the Guinness Book of World Records as the person with the longest entry in Who's Who in America.
Of Swedish, distant German and Belgian (Flemish and Walloon) ancestry, Seaborg was born in Ishpeming, Michigan, the son of Herman Theodore (Ted) and Selma Olivia Erickson Seaborg. He had one sister, Jeanette. When Glenn Seaborg was a boy, the family moved to the Seaborg Home in a subdivision called Home Gardens, that was later annexed to the City of South Gate, California, a suburb of Los Angeles.
He kept a daily journal from 1927 until he suffered a stroke in 1998. As a youth, Seaborg was both a devoted sports fan and an avid movie buff. His mother encouraged him to become a book keeper as she felt his literary interests were impractical. He did not take an interest in science until his junior year when he was inspired by Dwight Logan Reid, a chemistry and physics teacher at David Starr Jordan High School in Watts.
He graduated from Jordan in 1929 at the top of his class and received a bachelor's degree in chemistry at the University of California, Los Angeles, in 1933. While at UCLA, he was invited by his German professor to meet Albert Einstein,
an experience that had a profound impact on Seaborg and served as a
model of graciousness for his encounters with aspiring students in later
years. Seaborg worked his way through school as a stevedore (longshoreman), fruit packer and laboratory assistant.
Seaborg took his Ph.D. in chemistry at the University of California, Berkeley, in 1937 with a doctoral thesis on the inelastic scattering of neutrons in which he coined the term "nuclear spallation". He was a member of the professional chemistry fraternity Alpha Chi Sigma. As a graduate student in the 1930s Seaborg performed wet chemistry research for his advisor Gilbert Newton Lewis and published three papers with him on the theory of acids and bases. Seaborg then studied thoroughly the text Applied Radiochemistry by Otto Hahn, of the Kaiser Wilhelm Institute for Chemistry in Berlin and it had a major impact on his developing interests as a research scientist. For several years, Seaborg conducted important research in artificial radioactivity using the Lawrence cyclotron at UC Berkeley. He was excited to learn from others that nuclear fission was possible — but also chagrined, as his own research might have led him to the same discovery.
Seaborg also became expert in dealing with noted Berkeley physicist Robert Oppenheimer.
Oppenheimer had a daunting reputation, and often answered a junior
man's question before it had even been stated. Often the question
answered was more profound than the one asked, but of little practical
help. Seaborg learned to state his questions to Oppenheimer quickly and
succinctly.
Seaborg remained at the University of California, Berkeley, for post doctoral research. He followed Frederick Soddy's work investigating isotopes and contributed to the discovery of more than 100 isotopes of elements. Using one of Lawrence's advanced cyclotrons, John Livingood, Fred Fairbrother, and Seaborg created a new isotope of iron, iron - 59 (Fe-59) in 1937. Iron - 59 was useful in the studies of the hemoglobin in human blood. In 1938, Livingood and Seaborg collaborated (as they did for five years) to create an important isotope of iodine, iodine - 131 (I-131) which is still used to treat thyroid disease. (Many years later, it was credited with prolonging the life of Seaborg's mother.) As a result of these and other contributions, Seaborg is regarded as a pioneer in nuclear medicine and is one of its most prolific discoverers of isotopes.
In 1939 he became an instructor in chemistry at Berkeley, was promoted to assistant professor in 1941 and professor in 1945.
UC Berkeley physicist Edwin McMillan had led a team that discovered element 93, neptunium in 1940. In November 1940, McMillan was persuaded to leave Berkeley temporarily to assist with urgent research in radar technology. Since Seaborg and his colleagues had perfected McMillan's oxidation - reduction technique for isolating neptunium, he asked McMillan for permission to continue the research and search for element 94. McMillan agreed to the collaboration. Seaborg first reported alpha decay proportionate to only a fraction of the element 93 under observation. The first hypothesis for this alpha particle accumulation was contamination by uranium, which produces alpha - decay particles; analysis of alpha - decay particles ruled this out. Seaborg then postulated that a distinct alpha - producing element was being formed from element 93. In February 1941, Seaborg and his collaborators produced plutonium - 239 through the bombardment of uranium. This experimental achievement changed the course of human history in ways more profound than they could have ever imagined: the production of plutonium - 239 was successful. In their experiments bombarding uranium with deuterons, they observed the creation of neptunium, element 93. But it then underwent beta - decay, forming a new element, plutonium, with 94 protons. Plutonium is fairly stable, but undergoes alpha - decay, which explained the presence of alpha particles coming from neptunium. Thus, on March 28, 1941, Dr. Seaborg, physicist Emilio Segrè and Berkeley chemist Joseph W. Kennedy were able to show that plutonium (then known only as element 94239) underwent fission with slow neutrons, an important distinction that was crucial to the decisions made in directing Manhattan Project research. Room 307 of Gilman Hall on the campus at the University of California, Berkeley, where Seaborg did his work, has since been declared a U.S. National Historic Landmark.
In
addition to plutonium, he is credited as a lead discoverer of
americium, curium, and berkelium, and as a co-discoverer of californium,
einsteinium, fermium, mendelevium, nobelium and seaborgium. He shared
the Nobel Prize in Chemistry in 1951 with Edwin McMillan for
"their discoveries in the chemistry of the first transuranium
elements." He obtained patents on americium and curium, which were
developed in 1944 in Chicago at the wartime metallurgical laboratory
during the Manhattan project. His research contributions to all of the
other elements were conducted at the University of California, Berkeley.
On April 19, 1942, Seaborg reached Chicago, and joined the chemistry group at the Metallurgical Laboratory of the Manhattan Project at the University of Chicago, where Enrico Fermi and his group would later convert U238 to plutonium in the world's first controlled nuclear chain reaction using a chain reacting pile. Seaborg's role was to figure out how to extract the tiny bit of plutonium from the mass of uranium. Plutonium - 239 was isolated in visible amounts using a transmutation reaction on August 20, 1942 and weighed on September 10, 1942 in Seaborg's Chicago laboratory. He was responsible for the multi - stage chemical process that separated, concentrated and isolated plutonium. This process was further developed at the Clinton Engineering Works in Oak Ridge, Tennessee, and then entered full scale production at the Hanford Engineer Works, in Richland, Washington.
Seaborg's theoretical development of the actinide concept resulted in a redrawing of the Periodic Table of the Elements into its current configuration with the actinide series appearing below the lanthanide series. Seaborg developed the chemical elements americium and curium while
in Chicago. He managed to secure patents for both elements. His patent
on curium never proved commercially viable because of the element's
short half-life. Americium is commonly used in household smoke
detectors, however, and thus provided a good source of royalty income to
Seaborg in later years. Prior to the test of the first nuclear weapon,
Seaborg joined with several other leading scientists in a written
statement known as the Franck Report (secret
at the time but since published) calling on President Truman to conduct
a public demonstration of the atomic bomb witnessed by the Japanese
rather than engaging in a surprise attack. Truman instead proceeded to
drop two bombs, credited by most observers at the time with ending the
war, a uranium bomb on Hiroshima and a plutonium bomb on Nagasaki.
After the conclusion of World War II and the Manhattan Project, Seaborg was eager to return to academic life and university research free from the restrictions of wartime secrecy. In 1946, he added to his responsibilities as a professor by heading the nuclear chemistry research at the Lawrence Radiation Laboratory operated by the University of California on behalf of the United States Atomic Energy Commission. Seaborg was named one of the "Ten Outstanding Young Men in America" by the U.S. Junior Chamber of Commerce in 1947 (along with Richard Nixon and others). Seaborg was elected to the National Academy of Sciences in 1948. From 1954 to 1961 he served as associate director of the radiation laboratory. He was appointed by President Truman to serve as a member of the General Advisory Committee of the Atomic Energy Commission, an assignment he retained until 1960.
Seaborg served as chancellor at University of California, Berkeley from
1958 to 1961. His term as Chancellor came at a time of considerable
controversy during the time of the free speech movement. In October
1958, he announced that the University had relaxed its prior
prohibitions on political activity on a test basis. Seaborg served on the Faculty Athletic Committee for several years and is the co-author of a book concerning the Pacific Coast Conference scandal and the founding of the Pac-10 (formerly
Pac-8), in which he played a role. Seaborg served on the President's
Science Advisory Commission during the Eisenhower administration, which
produced the report "Scientific Progress, the Universities, and the
Federal Government," also known as the "Seaborg Report," in November
1960. The Seaborg Report is credited with influencing the federal policy
towards academic science for the next eight years. In 1959, he helped
found the Berkeley Space Sciences Laboratory with UC president Clark Kerr.
After appointment by President John F. Kennedy and confirmation by the United States Senate, Seaborg was chairman of the United States Atomic Energy Commission (AEC) from 1961 to 1971. His pending appointment by President Kennedy was nearly derailed in late 1960 when members of the Kennedy transition team learned that Seaborg had been listed in a U.S. News and World Report article as a member of "Nixon's Brain Trust." Seaborg said that as a lifetime Democrat he was baffled when the article appeared associating him with Vice President Nixon, whom he considered a casual acquaintance.
While chairman of the AEC, Seaborg participated on the negotiating team for the Limited Test Ban Treaty (LTBT). Seaborg considered his contributions to the achievement of the LTBT as his greatest accomplishment. Despite strict rules from the Soviets about photography at the signing ceremony, Seaborg sneaked a tiny camera past the Soviet guards to take a close-up photograph of Soviet Premier Nikita Khrushchev as he signed the treaty.
Seaborg
was ardent supporter of large scale massive nuclear plants for
electricity generation despite concerns by industry insiders that such
large plants were vulnerable in that their nuclear cores could not be
properly contained in the event of an accident or operating emergency.
Seaborg received a letter dated August 16, 1966 from industry engineers
expressing these concerns at the time of the licensing of New York's
Indian Point reactor. This letter advised Seaborg and other AEC senior
members of these containment concerns which would later become known as
the "China Syndrome" resulting from uncontained core meltdowns. Seaborg
directed this letter not be released to the public as he feared it would
be misunderstood and therefore damage the nuclear industry in the
public's view even though the law required such letters be released for
public disclosure. This disclosure first came to light for public view
in the BBC documentary series, "Pandora's Box, A Is For Atom" dealing
with the early history of commercial nuclear development.
Seaborg enjoyed a close relationship with President Lyndon Johnson and influenced the administration to pursue the Nuclear Non-Proliferation Treaty.
Seaborg was called to the White House in the first week of the Nixon Administration in January 1969 to advise President Richard Nixon on his first diplomatic crisis involving the Soviets and nuclear testing. Seaborg clashed with Nixon presidential adviser John Ehrlichman over the treatment of a Jewish scientist whom the Nixon administration suspected of leaking nuclear secrets to Israel.
Seaborg published several books and journal articles during his tenure at the Atomic Energy Commission. His predictions concerning development of stable super heavy elements are considered among his most important theoretical contributions. Seaborg theorized the transactinide series and the superactinide series of undiscovered synthetic elements. While most of these theoretical future elements have extremely short half-lives and thus no expected practical applications, Seaborg theorized an island of stability for isotopes of certain elements.
When
Seaborg resigned as chairman of the Atomic Energy Commission in 1971,
he had served longer than any other Kennedy appointee.
Following his service as Chairman of the Atomic Energy Commission, Seaborg returned to UC Berkeley where he was awarded the position of University Professor. At the time, there had been fewer University Professors at UC Berkeley than Nobel prize winners. He also served as Chairman of the Lawrence Hall of Science. Seaborg served as President of the American Association for the Advancement of Science in 1972 and as President of the American Chemical Society in 1976. In 1976, when the Swedish king visited the United States, Seaborg played a major role in welcoming the Swedish Royal Family.
In 1980, he transmuted several thousand atoms of bismuth into gold at the Lawrence Berkeley Laboratory. His experimental technique, using nuclear physics, was able to remove protons and neutrons from the bismuth atoms. Seaborg's technique would have been far too expensive to enable routine manufacturing of gold, but his work is the closest to the mythical Philosopher's Stone.
In 1983, President Ronald Reagan appointed Seaborg to serve on the National Commission on Excellence in Education. Upon seeing the final draft report, Seaborg is credited with making comments that it was far too weak and did not communicate the urgency of the current crisis. He compared the crisis in education to the arms race, and stated that we are "a nation at risk." These comments led to a new introduction to the report and gave the report the famous title which focused national attention on education as an issue germane to the federal government.
Seaborg lived most of his later life in Lafayette, California, where he devoted himself to editing and publishing the journals that documented both his early life and later career. He rallied a group of scientists who criticized the science curriculum in the State of California which he viewed as far too socially oriented and not nearly focused enough on hard science. California Governor Pete Wilson appointed Seaborg to head a committee that proposed sweeping changes to California's science curriculum despite outcries from labor organizations and others.
On August 24, 1998, while in Boston to attend a meeting by the American Chemical Society, Seaborg suffered a stroke, which led to his death six months later on February 25, 1999 at his home in Lafayette.
During
his lifetime, Seaborg is said to have been the author or co-author of
more than 50 books and 500 scientific journal articles, many of them
brief reports on fast- breaking discoveries in nuclear science while
other subjects, most notably the actinide concept, represented major
theoretical contributions in the history of science. He held more than 40 patents — among them the only patents ever issued for chemical elements, americium and curium.
He is also said to have received more than 50 degrees and honorary
degrees in his lifetime. At one time, he was listed in the Guinness Book of World Records as having the longest entry in Marquis Who's Who in America. In February 2005, Seaborg was posthumously inducted into the National Inventors Hall of Fame.
In 1942, Seaborg married Helen Griggs, the secretary of Ernest Lawrence.
Under wartime pressure, Seaborg had moved to Chicago while engaged to Griggs. When Seaborg returned to accompany Griggs for the journey back to Chicago, friends expected them to marry in Chicago. But, eager to be married, Seaborg and Griggs impulsively got off the train in the town of Caliente, Nevada, for what they thought would be a quick wedding. When they asked for City Hall, they found Caliente had none — they would have to travel 25 miles north to Pioche, the county seat. With no car, this was no easy feat but, happily, one of Caliente's newest deputy sheriffs turned out to be a recent graduate of the Cal Berkeley chemistry department and was more than happy to do a favor for Seaborg. The deputy sheriff arranged for the wedding couple to ride up and back to Pioche in a mail truck. The witnesses at the Seaborg wedding were a clerk and a janitor.
Glenn Seaborg and Helen Griggs Seaborg had six children, of whom the first, Peter Glenn Seaborg, died in 1997. The others were Lynne Seaborg Cobb, David Seaborg, Steve Seaborg, Eric Seaborg, and Dianne Seaborg.
Seaborg was an avid hiker. Upon becoming Chairman of the Atomic Energy Commission in 1961, he commenced taking daily hikes through a trail which he blazed at the headquarters site in Germantown, Maryland. He frequently invited colleagues and visitors to accompany him and the trail became known as the "Glenn Seaborg Trail."
He and his wife Helen are credited with blazing a 12 mile trail in the East Bay area near their Lafayette, California home. This trail has since become a part of the American Hiking Association's cross country network of trails. Seaborg and his wife walked the trail network from Contra Costa County all the way to the California - Nevada border.
Seaborg was honored as Swedish - American of the Year in 1962 by the Vasa Order of America. In 1991, the organization named "Local Lodge Glenn T. Seaborg No. 719" in his honor during the Seaborg Honors ceremony at which he appeared. This lodge maintains a scholarship fund in his name, as does the unrelated Swedish - American Club of Los Angeles.
Seaborg kept a close bond to his Swedish origin. He visited Sweden every so often and his family were members of the Swedish Pemer Genealogical Society, a family association open for every descendant of the Pemer family, a Swedish family with German origin, from which Seaborg was descended on his mother's side.
He was elected a foreign member of the Royal Swedish Academy of Sciences in 1972 and the Royal Society of London.
Seaborg was an enthusiastic supporter of Cal's sports teams. San Francisco columnist Herb Caen was fond of pointing out that Seaborg's surname is an anagram of "Go Bears", a popular cheer at UC Berkeley.
The element seaborgium was named after Seaborg by Albert Ghiorso, E. Kenneth Hulet, and others, who also credited Seaborg as a co-discoverer. It was so named while Seaborg was still alive, which proved controversial. He influenced the naming of so many elements that with the announcement of seaborgium, it was noted in Discover magazine's review of the year in science that he could receive a letter addressed in chemical elements: seaborgium, lawrencium (for the Lawrence Berkeley Laboratory where he worked), berkelium, californium, americium.
While
it has been commonly stated that seaborgium is the only element to have
been named after a living person, this is not entirely accurate; both einsteinium and fermium were proposed as names of new elements discovered by Albert Ghiorso while Enrico Fermi and Albert Einstein were still living. The discovery of these elements and their names were kept secret under Cold War era
nuclear secrecy rules, however, and thus the names were not known by
the public or the broader scientific community until after the deaths of
Fermi and Einstein. Thus seaborgium is the only element to have been
publicly named after a living person.

Edwin Mattison McMillan (September 18, 1907 – September 7, 1991) was an American physicist and Nobel laureate credited with being the first ever to produce a transuranium element. He shared the Nobel Prize in Chemistry with Glenn Seaborg in 1951.
McMillan was born in Redondo Beach, California, but his family moved to Pasadena the following year. He attended some of the public lectures at the California Institute of Technology as a high school student and began his studies there in 1924. He did a research project with Linus Pauling as an undergraduate and received his Bachelor of Science degree in 1928 and his Master of Science degree in 1929, both from the California Institute of Technology.
He then took his Doctor of Philosophy from Princeton University in 1932 for the thesis: "Deflection of a Beam of HCI Molecules in a Non - Homogeneous Electric Field" under the supervision of Edward Condon.
He joined the group of Ernest Lawrence at the University of California, Berkeley, upon receiving his doctorate, moving to the Berkeley Radiation Laboratory when it was founded at Berkeley in 1934.
His experimental skills lead to the discovery of oxygen - 15 with M. Stanley Livingston and beryllium - 10 with Samuel Ruben.
In 1940 he and Philip Abelson created neptunium, while conducting a fission experiment ofuranium - 239 with neutrons, using the cyclotron at Berkeley. The newly found isotope of neptunium was created by absorption of neutron into the uranium - 239 and a subsequent beta decay. McMillan understood the underlying principle of the reaction and started to bombard the uranium - 239 with deuterium to create the element 94. He moved to the radar research at the Massachusetts Institute of Technology and Glenn T. Seaborg finished the work.
In World War II, he was involved in research on radar at MIT in Cambridge, Massachusetts, sonar near San Diego, and about November 1942 was recruited to the Manhattan Project at the Los Alamos Laboratory, being involved in the initial selection of Los Alamos and in implosion research.
After World War II, he rejoined the Berkeley Radiation Laboratory, and became head of the institute after the death of Ernest Lawrence in 1958.
In 1945 he developed ideas for the improvement of the cyclotron, leading to the development of the synchrotron. The synchrotron was used to create new elements at Berkeley Radiation Laboratory extending the periodic system of elements far beyond the 92 elements known before 1940.
With Glenn T. Seaborg, he shared the Nobel Prize in Chemistry in 1951 for "discoveries in the chemistry of the transuranium elements." This medal is currently held at the National Museum of American History, a division of The Smithsonian.
In 1946, he became a full professor at Berkeley, and in 1954 he was appointed associate director of the Lawrence Radiation Laboratory, being promoted to director in 1958, where he stayed until his retirement in 1973.
He was elected to the National Academy of Sciences in 1947, serving as its chairman from 1968 to 1971. He received the Atoms for Peace Award in 1963.

Joseph William Kennedy (May 30, 1916 – May 5, 1957) was an American scientist credited with being a co-discoverer of plutonium along with Glenn T. Seaborg, Edwin McMillan, and Arthur Wahl.
Born in Nacogdoches, Texas, Kennedy attended Stephen F. Austin State Teachers College, the University of Kansas, and received his PhD at the University of California, Berkeley. In 1943, he arrived at the Los Alamos National Laboratory, and aided in the discovery, purification, and handling of plutonium.
In 1945, Kennedy was recruited as a professor at Washington University in St. Louis, and was installed as Chairman of the Department of Chemistry, a role he continued in until his death. Kennedy brought with him Wahl, Lindsay Helmholz, David Lipkin, Herbert Potratz, and Samuel Weissman, who all served on the faculty at Washington University.
Kennedy
died at the age of 40 after a battle with cancer, only two years after
Seaborg, McMillan, Wahl, and he received a prize of $400,000 dollars for
their scientific work.

Arthur C. Wahl (September 8, 1917 – March 6, 2006) was an American chemist who, as a PhD student of Glenn T. Seaborg at UC Berkeley, first isolated plutonium in February 1941. He also worked on the Manhattan Project.