<Back to Index>
- Inventor and Physicist Guillaume Amontons, 1663
- Inventor, Physicist and Balloonist Jacques Alexandre César Charles, 1746
- Chemist and Physicist Joseph Louis Gay - Lussac, 1778
PAGE SPONSOR

Guillaume Amontons (31 August 1663 – 11 October 1705) was a French scientific instrument inventor and physicist. He was one of the pioneers in tribology, along with Leonardo da Vinci, John Theophilus Desaguliers, Leonard Euler and Charles - Augustin de Coulomb.
Guillaume was born in Paris, France. His father was a lawyer from Normandy who had moved to the French capital. While still young, Guillaume lost his hearing, which may have motivated him to focus entirely on science. He never attended a university, but was able to study mathematics, the physical sciences, and celestial mechanics. He also spent time studying the skills of drawing, surveying and architecture.
He died in Paris, France.
He was supported in his research career by the government, and was employed in various public works projects.
Among his contributions to scientific instrumentation were improvements
to the barometer (1695), hygrometer (1687), and thermometer (1695),
particularly for use of these instruments at sea. He also demonstrated
an optical telegraph and proposed the use of his clepsydra (water clock) for keeping time on a ship at sea.
Amontons investigated the relationship between pressure and temperature in gases though he lacked accurate and precise thermometers. Though his results were at best semi - quantitative, he established that the pressure of a gas increases by roughly one-third between the temperatures of cold and the boiling point of water. This was a substantial step towards the subsequent gas laws and, in particular, Charles's law.
His work led him to speculate that a sufficient reduction in temperature would lead to the disappearance of pressure. Thus, he is the first researcher to discuss the concept of an absolute zero of temperature, a concept later extended and rationalized by William Thomson, 1st Baron Kelvin.
In 1699, Amontons published his rediscovery of the laws of friction first put forward by Leonardo da Vinci. Though they were received with some skepticism, the laws were verified by Charles - Augustin de Coulomb in 1781.
Amontons' Laws of Friction, first explored by Leonardo da Vinci but never published, were rediscovered and first recorded in print during the late 17th century.
There 3 laws of friction are:
- 1. The force of friction is directly proportional to the applied load. (Amontons 1st Law)
- 2. The force of friction is independent of the apparent area of contact. (Amontons 2nd Law)
- 3. Kinetic friction is independent of the sliding velocity. (Coulomb's Law)
NOTE: These 3 laws only apply to dry friction, in which the addition
of a lubricant modifies the tribological properties significantly.
By looking at any surface on the microscopic level, one would find that it is never perfectly flat. There would exist many tiny bumps and craters, due to imperfections on the surface and the alignment of molecules. (The skin does not feel the bumps and craters because they are too small to be detected.) Considering a smooth stone on a smooth flat road, the two surfaces would be still in contact, but only at a few points (the bumps do not fit exactly into the craters). Due to electrostatic forces of repulsion between the atoms (nuclei against nuclei and electrons against electrons) of the stone and the road, the road will exert a force on the stone, and the stone will exert a force on the road (normal contact forces). The force exerted on the stone would be the NORMAL contact force.
If an external force causes the stone to move to the RIGHT, the forces
that the road exert on the stone would be slightly skewed to the LEFT,
thus the net force from the road on the stone will be pointing UP but
LEFTWARD from the sum of all of the electrostatic forces (tilted contact
force). As the vertical component of the net force is the normal contact
force, the extra horizontal leftward component of the force would
therefore be the FRICTIONAL force. (Note: friction force OPPOSES sliding
of two surfaces in contact. On a macro level you could not walk forward
without friction pushing you forward)
Suppose the stone had a greater mass (hence greater weight as g=constant). The stone would then:
- exert a greater force on the road (the increased load causes the separation distance of the nuclei to decrease, force of repulsion becomes stronger (inverse - square law) ), AND
- more of the atoms of the road and the stone would be in contact.
Hence, when the stone is moved, a greater frictional force would be produced (more areas of contact means that more forces can be skewed, producing more horizontal components of the contact forces).
Amontons law applies to any 2 surfaces, regardless of their orientation (e.g. pressing a brick against the ceiling, etc.)
NOTE: Applied load means the normal contact force acting on
the stone. That is, if the stone is being pushed down harder while it
was trying to move, the force acting on the ground increases, and hence
the force of the ground acting on the stone (normal contact) increases.
This means that more force is required to move the stone across the
ground (frictional force increase).
What this law means is that if two equal masses made of similar material are resting on the same surface with different areas of contact, they would require the same amount of force to start moving (overcome static friction) and to move at constant speed.
To put it in another way: considering 2 equal masses, and the area in
contact in situation A is greater than in situation B. This only means
that in situation A, the load is distributed across a greater area than
in situation B. However, the applied load is still the same! Thus to move both masses, we would require the same amount of applied force to overcome friction (Amontons First Law).
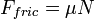 ,
,
where μ is the coefficient of friction and N is the normal contact force.
This is as predicted by Amontons' two laws, where Ffric depends only on the normal contact force (reaction pair of the applied load), and is independent of the surface area in contact.
However, exceptions to Amontons' Law have been observed in various
nanometric scenarios. For example, when two surfaces get close enough
such that molecular interactions and atomic forces come into play, the
two surfaces are attracted together and form what was known as 'negative
load'.

Jacques Alexandre César Charles (November 12, 1746 – April 7, 1823) was a French inventor, scientist, mathematician and balloonist.
Charles and the Robert brothers launched the world's first (unmanned) hydrogen filled balloon in August 1783; then in December 1783, Charles and his co-pilot Nicolas - Louis Robert ascended to a height of about 1,800 feet (550 m) in a manned balloon. Their pioneering the use of hydrogen for lift led to this type of balloon being named a Charlière (as opposed to a Montgolfière which used hot air).
Charles's law, describing how gases tend to expand when heated, was formulated by Joseph Louis Gay - Lussac in 1802, but he credited it to unpublished work by Jacques Charles.
Charles was elected to the Académie des Sciences, in 1793, and subsequently became professor of physics at the Conservatoire des Arts et Métiers.
Charles was born in Beaugency - sur - Loire in 1746, He married Julie
Françoise Bouchaud des Hérettes (1784 – 1817), a creole woman 37 years
younger than himself. Reportedly the poet Alphonse de Lamartine also
fell in love with her, and she was the inspiration for Elvire in his 1820 autobiographical Poetic Meditation
"Le Lac" ("The Lake"), which describes in retrospect the fervent love
shared by a couple from the point of view of the bereaved man. Charles
outlived her and died in Paris on April 7, 1823.
Charles conceived the idea that hydrogen would be a suitable lifting agent for balloons having studied the work of Robert Boyle's Boyle's Law which was published 100 years earlier in 1662, and of his contemporaries Henry Cavendish, Joseph Black and Tiberius Cavallo. He designed the craft and then worked in conjunction with the Robert brothers, Anne - Jean and Nicolas - Louis, to build it in their workshop at the Place des Victoires in Paris. The brothers invented the methodology for the lightweight, airtight gas bag by dissolving rubber in a solution of turpentine and varnished the sheets of silk that were stitched together to make the main envelope. They used alternate strips of red and white silk, but the discoloration of the varnishing / rubberizing process left a red and yellow result.
Jacques Charles and the Robert brothers launched the world's first hydrogen filled balloon on August 27, 1783, from the Champ de Mars, (now the site of the Eiffel Tower) where Ben Franklin was among the crowd of onlookers. The balloon was comparatively small, a 35 cubic meter sphere of rubberized silk, and only capable of lifting circa 9 kg (20 lb). It was filled with hydrogen that had been made by pouring nearly a quarter of a tonne of sulphuric acid onto a half a tonne of scrap iron. The hydrogen gas was fed into the balloon via lead pipes; but as it was not passed through cold water, great difficulty was experienced in filling the balloon completely (the gas was hot when produced, but as it cooled in the balloon, it contracted). Daily progress bulletins were issued on the inflation; and the crowd was so great that on the 26th the balloon was moved secretly by night to the Champ de Mars, a distance of 4 kilometers.
The balloon flew northwards for 45 minutes, pursued by chasers on
horseback, and landed 21 kilometers away in the village of Gonesse where
the reportedly terrified local peasants destroyed it with pitchforks or knives. The project was funded by a subscription organized by Barthelemy Faujas de Saint - Fond.
At 13:45 on December 1, 1783 Jacques Charles and the Robert brothers launched a new manned balloon from the Jardin des Tuileries in Paris. Jacques Charles was accompanied by Nicolas - Louis Robert as co-pilot of the 380 cubic meter, hydrogen filled balloon. The envelope was fitted with a hydrogen release valve and was covered with a net from which the basket was suspended. Sand ballast was used to control altitude. They ascended to a height of about 1,800 feet (550 m) and landed at sunset in Nesles - la - Vallée after a 2 hour 5 minute flight covering 36 km. The chasers on horseback, who were led by the Duc de Chartres, held down the craft while both Charles and Nicolas - Louis alighted.
Jacques Charles then decided to ascend again, but alone this time because the balloon had lost some of its hydrogen. This time it ascended rapidly to an altitude of about 3,000 meters, where he saw the sun again. He began suffering from aching pain in his ears so he 'valved' to release gas, and descended to land gently about 3 km away at Tour du Lay. Unlike the Robert brothers, Charles never flew again, although a hydrogen balloon came to be called a Charlière in his honor.
It is reported that 400,000 spectators witnessed the launch, and that hundreds had paid one crown each to help finance the construction and receive access to a 'special enclosure' for a "close - up view" of the take off. Among the 'special enclosure' crowd was Benjamin Franklin, the diplomatic representative of the United States of America. Also present was Joseph Montgolfier, whom Charles honored by asking him to release the small, bright green, pilot balloon to assess the wind and weather conditions.
This event took place ten days after the world's first manned balloon flight by Jean - François Pilâtre de Rozier using a Montgolfier brothers hot air balloon. Simon Schama wrote in Citizens:
| “ | Montgolfier's principal scientific collaborator was M. Charles, ... who had been the first to propose the gas produced by vitriol instead of the burning, dampened straw and wood that he had used in earlier flights. Charles himself was also eager to ascend but had run into a firm veto from the King, who from the earliest reports had been observing the progress of the flights with keen attentiveness. Anxious about the perils of a maiden flight, the King had then proposed that two criminals be sent up in a basket, at which Charles and his colleagues became indignant. | ” |
The next project of Jacques Charles and the Robert brothers was to build an elongated, steerable craft that followed Jean Baptiste Meusnier's proposals (1783 – 85) for a dirigible balloon. The design incorporated Meusnier's internal ballonnet (air cells), a rudder and a method of propulsion.
Jacques Charles chose never to fly in this craft, but on July 15, 1784 the brothers flew for 45 minutes from Saint - Cloud to Meudon with M. Collin - Hullin and Louis Philippe II, the Duke of Chartres in La Caroline. It was fitted with oars for propulsion and direction, but they proved useless. The absence of a 'gas release valve' meant that the duke had to slash the 'ballonnet' to prevent rupture when they reached an altitude of circa 4,500 metres (14,800 ft).
On September 19, 1784 the Robert brothers and M. Collin - Hullin flew for 6 hours 40 minutes, covering 186 km from Paris to Beuvry near Béthune. This was the first flight over 100 km.
Charles developed several useful inventions, including a valve to let
hydrogen out of the balloon and other devices, such as the hydrometer and reflecting goniometer, and improved the Gravesand heliostat and Fahrenheit's aerometer. In addition he confirmed Benjamin Franklin's electrical experiments.
Charles' law (also known as the law of volumes), describing how gases tend to expand when heated, was first published by natural philosopher Joseph Louis Gay - Lussac in 1802, but he credited it to unpublished work by Jacques Charles, and named the law in his honor.
Around 1787 Charles did an experiment where he filled 5 balloons to the same volume with different gases. He then raised the temperature of the balloons to 80 °C and noticed that they all increased in volume by the same amount. This experiment was referenced by Gay - Lussac in 1802 when he published a paper on the precise relationship between the volume and temperature of a gas. Charles' Law states that under constant pressure, an ideal gas' volume is proportional to its absolute temperature. The volume of a gas at constant pressure increases linearly with the absolute temperature of the gas. The formula he created was V1/T1 = V2/T2.
Charles was elected to the Académie des Sciences, in 1793, and subsequently became professor of physics at the Conservatoire des Arts et Métiers.
A stele at Nesles - la - Vallée marks the Charles - Robert flight of 1 December 1783.
The Coupe Charles et Robert was an international ballooning event that was run in 1983 in parallel with the Gordon Bennett Cup (ballooning).



Joseph Louis Gay - Lussac (also Louis Joseph Gay - Lussac, 6 December 1778 – 9 May 1850) was a French chemist and physicist. He is known mostly for two laws related to gases, and for his work on alcohol - water mixtures, which led to the degrees Gay - Lussac used to measure alcoholic beverages in many countries.
Gay - Lussac was born at Saint - Léonard - de - Noblat in the department of Haute - Vienne. He received his early education at the hands of the Catholic Abbey of Bourdeix. Later, in the care of the Abbot of Dumonteil he began his education in Paris, finally entering the École Polytechnique in 1798. Gay - Lussac narrowly avoided conscription and by the time of entry to the École Polytechnique his father had been arrested (due to Robespierre's Reign of Terror). Three years later, Gay - Lussac transferred to the École des Ponts et Chaussées, and shortly afterwards was assigned to C. L. Berthollet as his assistant. In 1802, he was appointed demonstrator to A. F. Fourcroy at the École Polytechnique, where in (1809) he became professor of chemistry. From 1808 to 1832, he was professor of physics at the Sorbonne, a post which he only resigned for the chair of chemistry at the Jardin des Plantes. In 1821, he was elected a foreign member of the Royal Swedish Academy of Sciences. In 1831 he was elected to represent Haute - Vienne in the chamber of deputies, and in 1839 he entered the chamber of peers.
Gay - Lussac married Geneviève - Marie - Joseph Rojot in 1809. He had first met her when she worked as a linen draper's shop assistant and was studying a chemistry textbook under the counter. He fathered five children, of whom the eldest (Jules) became assistant to Justus Liebig in Giessen. Some publications by Jules are mistaken as his father's today since they share the same first initial (J. Gay-Lussac).
Gay - Lussac died in Paris, and his grave is there at the Père Lachaise cemetery.
Some of Gay - Lussac's descendants live in Brazil, South America (de
Salusse Lussac / Lussac Do Coutto / Do Coutto Monni), and in Ontario, Canada.
- 1802 - Gay - Lussac first formulated the law, Gay - Lussac's Law, stating that if the mass and pressure of a gas are held constant then gas volume increases linearly as the temperature rises. This is sometimes written as V = k T, where k is a constant dependent on the type, mass, and pressure of the gas and T is temperature on an absolute scale. (In terms of the ideal gas law, k = n R / P.)
- 1804 - He and Jean - Baptiste Biot made a hot air balloon ascent to a height of 7016 meters (23,000 feet) in an early investigation of the Earth's atmosphere. He wanted to collect samples of the air at different heights to record differences in temperature and moisture.
- 1805 - Together with his friend and scientific collaborator Alexander von Humboldt, he discovered that the composition of the atmosphere does not change with decreasing pressure (increasing altitude). They also discovered that water is formed by two parts of hydrogen and one part of oxygen (by volume).
- 1808 - He was the co-discoverer of boron.
- 1810 - In collaboration with Louis Thenard, he developed a method for quantitative elemental analysis by measuring the CO2 and O2 evolved by reaction with potassium chlorate.
- 1811 - Gay - Lussac recognized iodine as a new element, described its properties, and suggested the name iode.
- 1824 - He developed an improved version of the burette that included a side arm, and coined the terms "pipette" and "burette" in an 1824 paper about the standardization of indigo solutions.
- In Paris, a street and a hotel near the Sorbonne are named after him as are a square and a street in his birthplace, Saint - Léonard - de - Noblat.