<Back to Index>
- Engineer and Physicist Benoît Paul Émile Clapeyron, 1799
- Physicist John James Waterston, 1811
- Physicist and Mathematician Rudolf Julius Emanuel Clausius, 1822
- Chemist and Physicist August Karl Krönig, 1799
PAGE SPONSOR

Benoît Paul Émile Clapeyron (26 February 1799 – 28 January 1864) was a French engineer and physicist, one of the founders of thermodynamics.
Born in Paris, Clapeyron studied at the École polytechnique and the École des Mines, before leaving for Saint Petersburg in 1820 to teach at the École des Travaux Publics. He returned to Paris only after the Revolution of July 1830, supervising the construction of the first railway line connecting Paris to Versailles and Saint - Germain. He married Mélanie Bazaine, daughter of Pierre - Dominique Bazaine (mathematician and bridge and canal engineer), and older sister of Pierre - Dominique (Adolphe) Bazaine (railway engineer) and Francois Achille Bazaine (Marshal of France).
In 1834, he made his first contribution to the creation of modern thermodynamics by publishing a report entitled the Driving force of the heat (Puissance motrice de la chaleur), in which it developed the work of the physicist Nicolas Léonard Sadi Carnot, deceased two years before. Though Carnot had developed a compelling analysis of a generalized heat engine, he had employed the clumsy and already unfashionable caloric theory.
Clapeyron, in his memoire, presented Carnot's work in a more accessible and analytic graphical form, showing the Carnot cycle as a closed curve on an indicator diagram, a chart of pressure against volume (named in his honor Clapeyron's graph).
In 1843, Clapeyron further developed the idea of a reversible process, already suggested by Carnot and made a definitive statement of Carnot's principle, what is now known as the second law of thermodynamics.
These foundations enabled him to make substantive extensions of Clausius' work, including the formula, now known as the Clausius – Clapeyron relation, which characterises the phase transition between two phases of matter. He further considered questions of phase transitions in what later became known as Stefan problems.
Clapeyron also worked on the characterization of perfect gases, the equilibrium
of homogeneous solids, and calculations of the statics of continuous
beams, notably the theorem of three moments (Clapeyron's theorem).

John James Waterston (1811 – 18 June 1883) was a Scottish physicist, a neglected pioneer of the kinetic theory of gases.
Waterston's father, George, was an Edinburgh sealing wax manufacturer and stationer, a relative of the Sandeman family Robert and his brother, George. John was born, the sixth of nine children, into a family alive with interests in literature, science and music. He was educated at Edinburgh High School before becoming apprenticed as a civil engineer to Messrs. Grainger and Miller. His employers encouraged him to attend lectures at the University of Edinburgh. He studied mathematics and physics under Sir John Leslie as well as attending lectures in chemistry, anatomy and surgery and becoming an active participant in the student literary society.
At age nineteen, Waterston published a paper proposing a mechanical explanation of gravitation, accounting for action at a distance in terms of colliding particles and discussing interactions between linear and rotational motion that would play a part in his later kinetic theory.
Waterston moved to London at age twenty - one, where he worked as a railroad surveyor,
becoming an associate of the Institution of Civil Engineers and
publishing a paper on a graphical method for planning earthworks.
The travel and disruption associated with his surveying work left
Waterston little time to pursue his studies so he joined the hydrography
department of the Admiralty under Francis Beaufort. It was Beaufort
who, in 1839, supported Waterston for the post of naval instructor for
cadets of the East India Company in Bombay. The posting worked well for Waterston who was able to pursue his reading and research at the library of Grant College.
While in India, he first developed his kinetic theory, independently
of earlier and equally neglected partial accounts by Daniel Bernoulli
and John Herapath. He published it, at his own expense, in his book Thoughts on the Mental Functions
(1843). He correctly derived all the consequences of the premise that
gas pressure is a function of the number of molecules per unit volume, N; molecular mass, M; and molecular mean-squared velocity, ![]() . He established the relationship:
. He established the relationship:
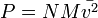 .
.
He had been motivated to think of a wave theory of heat by analogy with the wave theory of light and some experiments by James Forbes and Macedonio Melloni on radiant heat. His statement that ... in mixed media the mean square molecular velocity is inversely proportional to the specific weight of the molecules has been seen as the first statement of the equipartition theorem for translational motion. Waterston grasped that, while the kinetic energy of an individual molecule with velocity v is ½mv², heat energy is proportional to temperature, T. That insight led him to derive the ideal gas law:
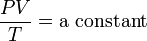 .
.
The publication made little impact, perhaps because of the title. He submitted his theory, under Beaufort's sponsorship, to the Royal Society in 1845 but was rejected. Referee Sir John William Lubbock wrote The paper is nothing but nonsense.
Unable to retrieve a copy of his paper (he had failed to make a copy
for himself before submitting the paper to the Royal Society), he
rewrote the work and sought to advertise it elsewhere, attracting little
attention other than from William John Macquorn Rankine and Hermann von
Helmholtz through whom it may have influenced August Krönig. The theory
gained acceptance only when it was proposed by Rudolf Clausius and
James Clerk Maxwell in the 1850s by which time Waterston's contribution
had been forgotten.
He returned to Edinburgh in 1857 to pursue his own novel physical ideas but met with unyielding neglect and discouragement from the scientific establishment. Neglect was exacerbated by his own increasing reclusiveness and hostility to the learned societies. He worked on acoustics, astronomy, fluid mechanics and thermodynamics.
He left his Edinburgh home on 18 June and drowned in a nearby canal, possibly falling into the canal due to heat stress from his astronomical observation activities.
As discussed above, Waterston's paper submitted to the Royal Society was rejected. Some years after Waterston's death, Lord Rayleigh (Secretary of Royal Society at that time) managed to dig it out from the archives of the Royal Society. Finally, Waterson's paper was published in the Philosophical Transactions of the Royal Society in 1892.

Rudolf Julius Emanuel Clausius (Born Rudolf Gottlieb, 2 January 1822 – 24 August 1888), was a German physicist and mathematician and is considered one of the central founders of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he put the theory of heat on a truer and sounder basis. His most important paper, On the mechanical theory of heat, published in 1850, first stated the basic ideas of the second law of thermodynamics. In 1865 he introduced the concept of entropy.
Clausius was born in Köslin (now Koszalin) in the Province of Pomerania. He started his education at the school of his father. After a few years, he went to the Gymnasium in Stettin (now Szczecin). Clausius graduated from the University of Berlin in 1844 where he studied mathematics and physics with, among others, Gustav Magnus, Johann Dirichlet and Jakob Steiner. He also studied history with Leopold von Ranke. During 1847, he got his doctorate from the University of Halle on optical effects in the Earth's atmosphere. He then became professor of physics at the Royal Artillery and Engineering School in Berlin and Privatdozent at the Berlin University. In 1855 he became professor at the ETH Zürich, the Swiss Federal Institute of Technology in Zürich, where he stayed until 1867. During that year, he moved to Würzburg and two years later, in 1869 to Bonn.
In 1870 Clausius organized an ambulance corps in the Franco - Prussian War. He was wounded in battle, leaving him with a lasting disability. He was awarded the Iron Cross for his services.
His wife, Adelheid Rimpham, died in childbirth in 1875, leaving him to raise their six children. He continued to teach, but had less time for research thereafter.
In 1886 he remarried Sophie Sack, and then had another child.
Two years later, on 24 August 1888, he died in Bonn, Germany.
Clausius' PhD thesis concerning the refraction of light proposed that we see a blue sky during the day, and various shades of red at sunrise and sunset (among other phenomena) due to reflection and refraction of light. Later, Lord Rayleigh would show that it was in fact due to the scattering of light, but regardless, Clausius used a far more mathematical approach than some have used.
His most famous paper, "Über die bewegende Kraft der Wärme" ("On the Moving Force of Heat and the Laws of Heat which may be Deduced Therefrom") was published in 1850, and dealt with the mechanical theory of heat. In this paper, he showed that there was a contradiction between Carnot's principle and the concept of conservation of energy. Clausius restated the two laws of thermodynamics to overcome this contradiction (the third law was developed by Walther Nernst, during the years 1906 – 1912). This paper made him famous among scientists.
During 1857, Clausius contributed to the field of kinetic theory after refining August Krönig's very simple gas - kinetic model to include translational, rotational and vibrational molecular motions. In this same work he introduced the concept of 'Mean free path' of a particle.
Clausius deduced the Clausius – Clapeyron relation from
thermodynamics. This relation, which is a way of characterizing the
phase transition between two states of matter such as solid and liquid,
had originally been developed in 1834 by Émile Clapeyron.
In 1865, Clausius gave the first mathematical version of the concept of entropy, and also gave it its name. He used the now abandoned unit 'Clausius' (symbol: Cl) for entropy. Clausius chose the word "entropy" because the meaning, from Greek, en+tropein, is "content transformative" or "transformation content" ("Verwandlungsinhalt").
- 1 Cl = 1 cal/°C = 4.1868 joules per kelvin (J/K)
- He was elected a Fellow of the Royal Society of London in 1868 and received its Copley Medal in 1879.
- He was elected a member of the Royal Swedish Academy of Sciences in 1878.
- He received the Huygens Medal in 1870.
- He received the Poncelet Prize in 1883.
- He received an honorary doctorate from the University of Würzburg in 1882.
- The crater Clausius on the Moon is named in his honor.
The following are two famous quotes made by Clausius in 1865:
The energy of the universe is constant.
The entropy of the universe tends to a maximum.
August Karl Krönig (1822 – 1879) was a German chemist and physicist who published an account of the kinetic theory of gases in 1856, probably after reading a paper by John James Waterston.
Krönig was born in Schildesche, now part of Bielefeld. After completing his Abitur he attended the University of Bonn for three semesters beginning in 1839 studying primarily Oriental languages. In 1840 he changed focus to physics, chemistry and mathematics and transferred to the University of Berlin, where he completed his doctorate in 1845 with a thesis on chromate salts. He then taught at two schools in Berlin, the Realgymnasium in Cölln and the Königliche Realschule attached to the Friedrich - Wilhelms - Gymnasium in Berlin. In 1864 he was given a professorship, but he was forced to retire the same year for reasons of ill health.
In 1851 Krönig self published the Journal für Physik und physikalische Chemie des Auslandes in vollständigen Übersichten, but only one year of issues appeared. In 1856 he published a paper on the kinetic theory of gases, thereby becoming a pioneer of statistical mechanics and thermodynamics alongside Rudolf Clausius, James Clerk Maxwell and Ludwig Boltzmann. The paper echoes John James Waterston's 1851 paper on the topic, and may be based on it; Clausius' work was sparked by Krönig's paper but emphasized the implication of "equal volumes equal numbers", which Waterston and Krönig merely noted. Krönig also published various writings on science and on philosophy and theology.
He died in Berlin; his remains were reburied in the family grave at the Südwestkirchhof Stahnsdorf, in Stahnsdorf, near Berlin.